8 Facts Every Medical Software Developer Should Know About the MDR
From 26 May 2021 (originally planned for 26 May 2020) onwards the Medical Device Regulation (MDR) will replace the current
Behind every scientific breakthrough is a story — one that connects data to decisions, research to recognition, and innovation to real impact. At MedTextpert, we believe that communicating science clearly and creatively is just as important as the discovery itself.
Our blog dives into the world of medical writing, scientific communication, and digital strategy — exploring how to make complex ideas understandable and engaging. From crafting accurate, compliant manuscripts to boosting visibility through compelling visuals, GEO/SEO, omnichannel content, and the thoughtful use of AI, we share insights to help you reach your audiences with clarity and confidence.
Whether you work in MedTech, Pharma, or Diagnostics, you’ll find practical tips, expert perspectives, and fresh ideas that turn complexity into clarity — and knowledge into impact.

From 26 May 2021 (originally planned for 26 May 2020) onwards the Medical Device Regulation (MDR) will replace the current
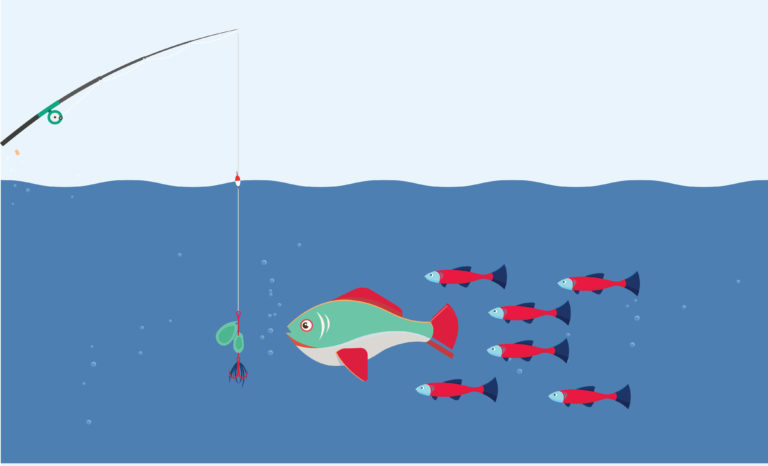
Inbound Marketing is a bit like fishing. Why? To fully understand the process you need to understand its components –
Content marketing is arguably the hottest topic in today’s landscape. And the opportunities are plentiful, from blogs to ebooks, podcasts to

We have learned in previously why an emotional approach in B2B marketing is helping to strengthen brand experiences. And we have learned

When thinking of B2B marketing, most people conjure an image of rational, fact-based materials, crafted in a clear cut, professional
Customer testimonials are an extremely powerful tool for businesses. We all know that. Before buying a new gadget from Amazon,