Conflict of interest (COI) disclosure by authors of medical articles is a required standard of most medical journals. However, our experience shows that it is still perceived as inconvenient and even annoying, causing extra work on top. And it is often done halfhearted, particularly if there are no specific instructions or forms for reporting COIs provided by the target journal. Especially authors that don’t publish on a regular base are not always aware of available resources that help declare conflicts of interest “correctly”.
This post is intended to raise awareness, provide an overview of the different forms of COIs, gives examples of what to disclose, and provides links to further information.
Conflicts of Interest are still underreported
As everyone can imagine, underreporting COIs is still common for various reasons.
In a recent study, Hakoum et al. [1] assessed the frequency and types of conflicts of interest disclosed by authors of primary studies of health policy and systems research (HPSR). Of 200 eligible studies, only 132 (66%) included COI disclosure statements. In 19 (14%) cases, at least one author reported one type of COI, with individual financial COI being the most frequently reported type (n=15, 11%). In general, financial and personal COIs were reported more frequently compared with non-financial and institutional COIs. Full article here.
Benea et al. [2] investigated the extent to which pharmaceutical industry funding and author-industry funding conflict of interests were declared for drug trials included in meta-analyses published in high-impact journals. Among 29 meta-analyses reviewed, only 13 listed the funding source of the included trials. In 2009 only 2 (of 29) were found. Although the number has increased, it is still suboptimal and gives an idea of the number of unreported cases.

Fig 1: Screenshot of JAMA Special Issue on COI in Medical Journal.
In 2017 JAMA [3] dedicated an entire issue to the topic of COI in Medical Journals.
Just looking at the section titles gives a good impression of the complexity of the case. Furthermore, it illustrates that not only authors or industry are concerned but also editors, publishers, journals, academic institutions, and of course, patients.
What is a Conflict of Interest in Medical Research
According to Lo B and Field MJ (eds) [5] is a Conflict of Interest typically defined as “a set of circumstances that creates a risk that professional judgment or actions regarding a primary interest will be unduly influenced by a secondary interest.” The primary interests include “promoting and protecting the integrity of research, the welfare of patients, and the quality of medical education;” and secondary interests comprise “not only financial gain, but also the desire for professional advancement, recognition for personal achievement, and favors to friends and family or students and colleagues.” Link here.
In the context of medical publications, not only authors and investigators but also reviewers, editors and publishers, and even institutions that are affiliated to the author might have a specific interest that could affect the integrity of the research.
Which COIs are relevant to publishing research findings
Regarding Conflicts of Interest, two categories are usually chosen: financial and non-financial. However, often the non-financial are not really considered essential and therefore not reported, as they are challenging to measure.
One could also imagine categorizing individual and institutional benefits first before determining sub-categories such as financial (direct and indirect), intellectual, personal (career, social status, political or religious beliefs, legal) – see our illustration for a possible categorization.
Examples of conflicts of interest
Following, we have compiled examples that could potentially jeopardize the research integrity of your manuscript if not disclosed.
Individual conflicts of interest include:
- Personal fees (e.g., consulting fees, fees for presentations, lectures, or educational events)
- Payments for manuscript writing, article processing charges, open access
- Gifts received (e.g., samples, equipment, tools, or software)
- Benefits related to product launch and promotion (e.g., meet the expert sessions, product testimonials)
- Sponsored travel
- Grants or funding received
- Intellectual property (e.g., ownership in patents, copyrights, royalties)
- Stock options or shared ownership
- Collaborations with external special interest groups – paid or voluntary
- Board positions held
- Previous or actual professional relationships (e.g., with reviewers, editors, publishers)
- Other competing interests (e.g., academic, political, religious, legal, personal)
*One more word to personal competing interests, this also includes family members that contributed to the work or are collaborating.
Institutional conflicts of interest include:
- Grants or funding received
- Support of related research projects
- Intellectual property (e.g., ownership in patents, copyrights)
- Spin-offs or start-ups that are founded based on research and development work
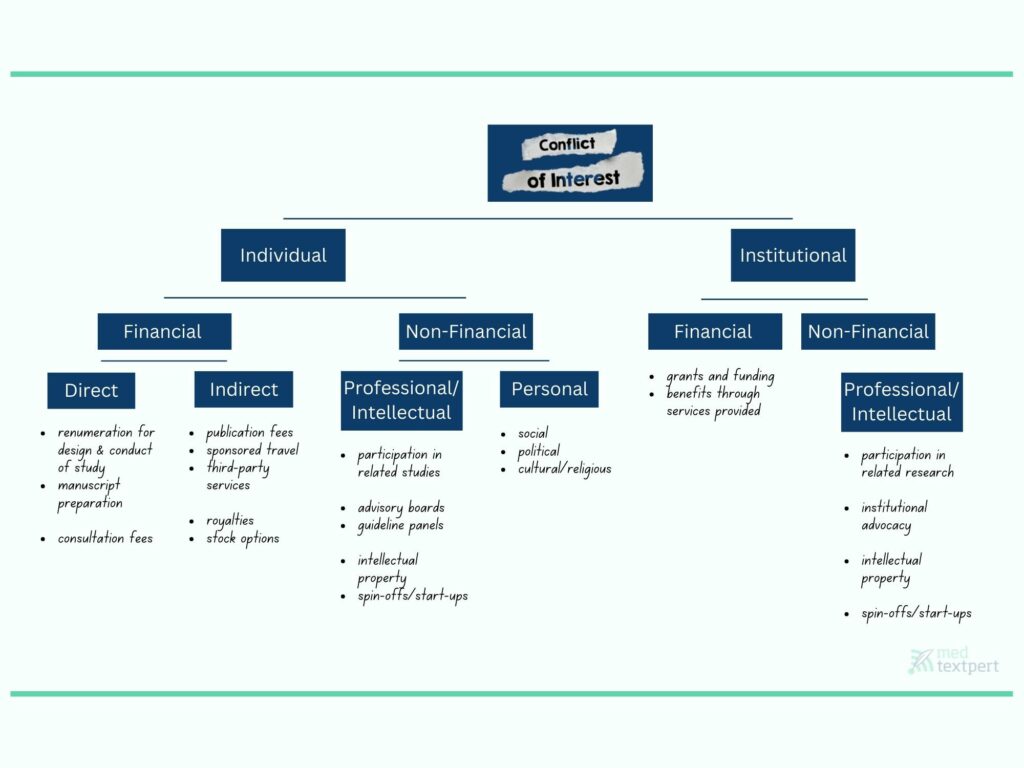
Fig 2: Possible COI categorization and examples
Looking at the examples, especially authors and investigators who work closely with the industry would profit from a fixed set of disclosing rules.
The reporting of COI should be standardized
Over the last decade, many journals have implemented a standardized publication process. They provide, among others, a set of instructions for manuscript preparation, submission checklists, and of course, require to declare the author’s interests related to the work.
However, a sentence like “All authors must disclose any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work“ is often all that is provided – a reminder.
But what exactly should be disclosed, and which timeframe is concerned?
GPP Guidelines
In 2015 the Good Publication Practice for communicating company-sponsored medical research: GPP3 guideline was published by the International Society of Medical Publication Professionals.[5]
It includes clarification and explicitly points out to fully disclose
- the role of the sponsor in the design, execution, analysis, reporting, and funding (if applicable) in all publications and presentations of the findings.
- any involvement by persons or organizations with interest (financial or non-financial) in the findings
- any relationships or potential competing interests relating to the research and its publication or presentation for authors and contributors.
Click here to access the whole article in the Annals of Internal Medicine.

Did you know that the newest update, the 2022 update of the Good Publication Practice (GPP 2022) guidelines, was just published in the Annals of Internal Medicine on August 30, 2022? Access here.
ICMJE Disclosure Form
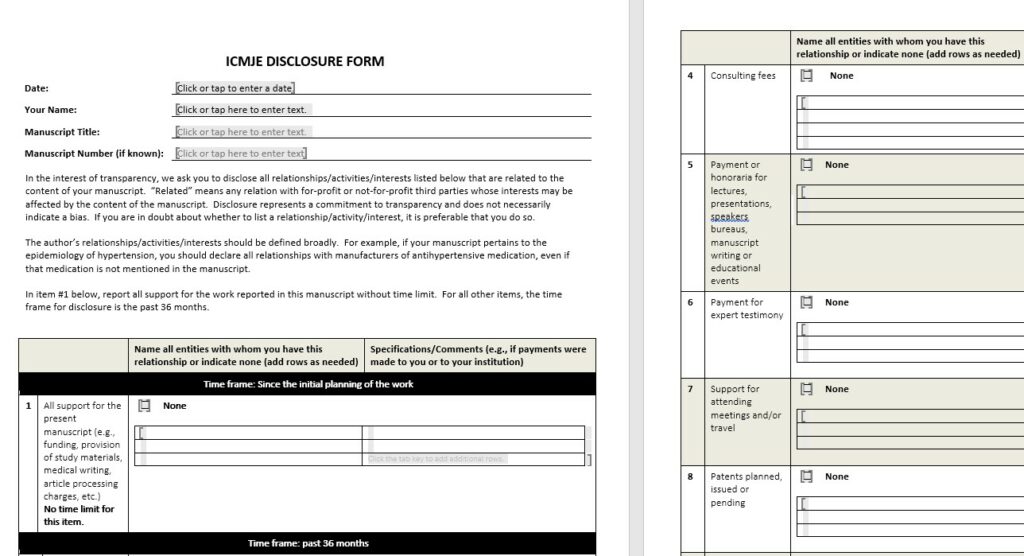
Fig 4: Screenshot of ICMJE Disclosure Form
The best thing is, that this form can be used free of charge for journal submissions where detailed instructions or forms are absent. It can be directly downloaded on their website (follow the link here).
Also, the International Committee of Medical Journal Editors (ICME), a small group of general medical journal editors, provides “Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals.”
Current Members of the ICMJE include renowned journals like The Lancet and JAMA.
ICMJE also offers a general Disclosure Form to promote standardization of disclosures. All member journals use this form in their submission process. The time frame for reporting is suggested to include 36 months prior to manuscript submission.
Elsevier provides a Guide to COI and how to prevent it; see screenshot.
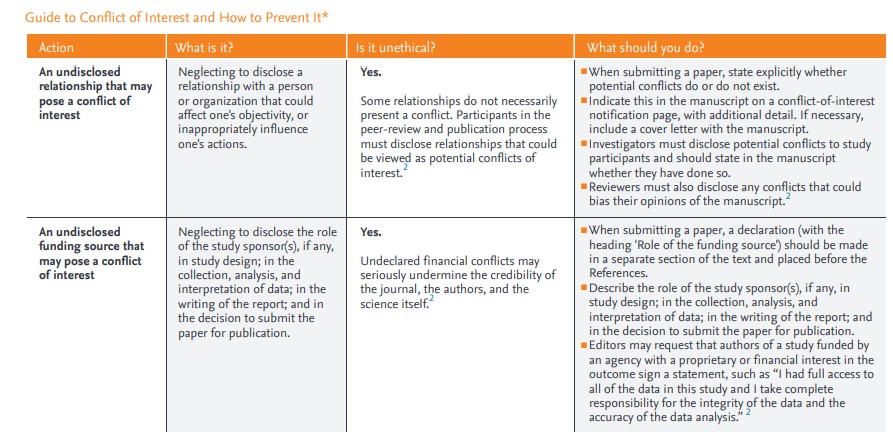
Fig 5: Screenshot of Elsevier’s COI guide. Access here.
As said before, failure to disclose a COI can lead to the retraction of a paper, as the peer review process is or has likely been compromised. Publication bans are possible, but usually, papers are corrected. Non-disclosure incidents harm academic careers, damage an organization’s reputation and shed a dim light on the peer review process and the journal’s credibility.
Example of not reporting COIs
Probably the most prominent example of non-declaration of various conflicts of interests at present, freely accessible via this link (page 66).

Fig 6: Screenshot of page 67/68 of NEJM disclosure form – Ugur Sahin
This example calls into question the data integrity of the study but also casts dark, politically motivated shadows on the publication practices of the New England Journal of Medicine.
Take home message
The transparency with which an author’s relationships and activities, directly or indirectly related to work, are handled during the planning, implementation, writing, peer review, editing, and publication of scientific work affects public trust in the scientific process and the credibility of published articles.
Only partly or not at all disclosed potential Conflicts of Interests cannot only lead to the retraction of the article but damage your or your institution’s reputation.
Methods and tools that verify the accuracy and completeness of declared COIs will be in place, especially with the help of artificial intelligence.
But also, journals will take on their responsibility to establish standardized COI disclosure rules that are not only focused on financial motives but include motives such as the desire for professional advancement or the willingness to do favors for family, friends, and colleagues.
“Having a conflict of interest is not in itself unethical, and some are unavoidable. Full transparency is always the best course of action, and if in doubt, disclose.” [6]
REFERENCES
[1] Hakoum MB, Bou-Karroum L, Al-Gibbawi M, et al. Reporting of conflicts of interest by authors of primary studies on health policy and systems research: a cross-sectional survey. BMJ Open. 2020;10(7):e032425. Published 2020 Jul 19. doi:10.1136/bmjopen-2019-032425
[2] Benea C, Turner KA, Roseman M, et al. Reporting of financial conflicts of interest in meta-analyses of drug trials published in high-impact medical journals: comparison of results from 2017 to 2018 and 2009. Syst Rev. 2020;9(1):77. Published 2020 Apr 8. doi:10.1186/s13643-020-01318-5
[3] Fontanarosa P, Bauchner H. Conflict of Interest and Medical Journals. JAMA. 2017;317(17):1768–1771. doi:10.1001/jama.2017.4563
[4] Lo B, Field MJ (eds) (2009) Conflict of interest in medical research, education, and practice. Institute of Medicine (US) Committee on conflict of interest in medical research, education, and practice. National Academies Press (US), Washington (DC)
[5] Battisti WP, Wager E, Baltzer L, et al. Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3. Ann Intern Med. 2015;163(6):461-464. doi:10.7326/M15-0288
[6] Office of Research Integrity U.S. Department of Health and Human Services. A brief overview on Conflict of Interests.